|
†††††††††† Last update: November 7, 2024 |
|
Carbon monoxide measurements while using a stove in a tent
A recent reference to a 1978 article in Backpacker Magazine titled New Research Proves Backpacking Stoves More Deadly Than Suspected† regarding the use of a stove in a tent prompted me to investigate for myself. Iíve used my 1973 Optimus 99 in my tent, all seasons for perhaps 1500 days/nights over many years. Itís a necessity for me if I want to go out at all because of rather severe Raynaudís Disease where always keeping warm is critical. Iíve been aware of the CO risk from the start but I finally decided to make some measurements instead of trying to extrapolate from old data. The following is based on a late October 2021 trip to Snow Lake in the Cascades with my sister Jane plus some experiments in the shop. Background: Carbon monoxide (CO) preferentially bonds to hemoglobin in red blood cells with an affinity 240 times that of oxygen. The result is the formation of carboxyhemoglobin (COHb) which binds up hemoglobin such that it cannot transport oxygen.† At the point at which CO absorption stops, COHb levels will drop with a half-life of about 5 hours, meaning that after 5 hours the level is reduced to Ĺ, after 10 hours, ľ and so on. The effect of CO is similar to that of anemia; it reduces the ability of the body to deliver oxygen to cells via the bloodstream. Carbon monoxide also acts to some extent as a direct cellular poison. Carbon monoxide poisoning is usually thought of as acute or chronic. Exposure in a tent would typically be acute in nature and thus the province of emergency medicine. Making a presumptive diagnosis of CO poisoning relies in part on a high index of suspicion. Making a definitive diagnosis in less than clinically obvious cases is difficult because arterial blood levels of COHb may have been already lowered in the field by use of high flow oxygen, or there may be complicating factors like chronically high levels associated with smoking. In short, trying to conclusively prove CO poisoning from using a stove in a tent would be difficult unless those affected were severely ill. Occupational Safety and Health Administration (OSHA) regulations: ∑††††† OSHA enclosed space, 8 hour average level: 50 ppm maximum ∑††††† OSHA exposure limit: 100 ppm.† None of the measurements in the field were over 100 so this limit does not apply. Rather than trying to predict COHb levels based on measured ppm values, Iíll try a simpler approach. I can assume that the OSHA 8 hour average limit would apply to a typical work week and that exposures below 50 ppm averaged over 8 hours can be taken as reasonably safe. I can also take the daily maximum dose of CO as 50 ppm times 8 hours† or 400 ppm-hours or 24000 ppm-minutes. In what follows I can then compare this later value with the recorded maximum CO level in ppm times the number of minutes of exposure.† The result should be a conservative estimate of the percentage of the maximum CO exposure allowed by OSHA regulations.† Since Iím using maximum values, the real exposure will be lower. The setup: We measured CO levels at nose level within 8 inches of the face in three scenarios (lunch, dinner and early morning) using a Cheffort Carbon Monoxide Meter Model AS8700A. Typically the Max mode was used for each of the readings† in order to represent the worst possible case. With this mode,† the maximum value sensed since the mode was initiated is displayed. The image below shows the CO meter hung on the tent clothesline for taking readings at face level. The elevation at the test site was 4100 feet. What follows should not be applied to high elevations where less oxygen is available for metabolism. |

|
In one test, a Optimus Polaris was used in a Big Agnes tent modified for winter and closed up as it would be when snow camping. The Big Agnes tent with Chair Peak in the background:
In the other test, an Optimus 99 was used in a homemade tent with a zip-in vinyl window closed as it would be in the depth of winter as shown below. (Temperatures for these tests were from low 40s to 31 deg F in the morning.) This tent has a zippered vent at the back as well as adjustable ventilation at the top of the vinyl window. The rainfly is typically 4 to 6 inches away from the uncoated and breathable ripstop nylon tent body. Even after a snowstorm where the snow buries the sides of the rainfly, ventilation is preserved front and back. This would not be the case in many newer tents where ventilation might be greatly restricted in such circumstances. What follows in those cases may not apply. Both of the stoves have recently been completely disassembled and cleaned and both were operating as if new.
The zip in vinyl window, closed: |

|
The location of the stove: |

|
Showing how an overnight snowstorm can bury the sides of a rainfly. Although not shown in the photo, there is still adequate ventilation under the rainfly at the front and back of the tent. |

|
What I found: In the interest of brevity, Iíll try to summarize what we found. There is a representative chart showing data from the Optimus 99 at the end of the article. Priming the stove: While priming with the custom primer mix we use (alcohol stove fuel saturated with white gas), maximum CO levels averaged around 70 ppm over 4 runs. The Optimus 99 takes 2 minutes to prime, so the CO dose in ppm-minutes as discussed above is 140 ppm-minutes. This is about 0.6% of the OSHA maximum 8 hour dose, calculated as above. Using the stoves for heat, no pot: Optimus 99 maximum values were typically around 20 ppm. The values we have for the Optimus Polaris were about 30 ppm. The worst case scenario I have encountered here in the Cascades occurs when temps are consistently below 10 deg F and I may have to use the stove for heat for perhaps a total of 3 hours on an overnight trip.† The CO produced in this circumstance would be ~15% of the OSHA maximum 8 hour dose. I conclude that neither priming nor using the Optimus 99 for heat *with my setup* risks clinically significant CO poisoning. Using the stove to heat water or melt snow: With 1.5 cups of water in the Optimus 99 stove cap,† CO levels† gradually rose to a maximum of 85 to 89.† The Optimus Polaris had similar values. The 99 took about 4 minutes to boil the 1.5 cups of water, the usual amount I need for a meal. This is about 1.5% of the OSHA maximum 8 hour dose which does not seem that problematic. Melting snow for water may or may not present a more troublesome issue. I typically allow 45 minutes to melt snow although some of that time is getting set up, letting the stove warm up, pouring water from the melting pot into the storage container, etc. Iíll take the worst case: 90 ppm for 45 minutes. This is about 17% of the OSHA maximum 8 hour dose. Whether this is of concern is a personal decision. Increased CO production is presumably the result the flame being cooled where it impinges on the cold pot as shown below: |
|
Using pot raisers: As was discussed in the Backpacker Magazine article, raising the pot can greatly reduce the CO generated by the stove. In shop tests with the Optimus 99, raising the pot 1 inch resulted in CO levels in the same range as those without the pot. Boiling time increased slightly but in the greater scheme of things, itís probably not significant. Stability may be of concern so extra care will be needed so as not to spill hot water.† The pot raisers shown below were made from 1/8 inch music wire with small brass silver-soldered retainers to hold the risers in place. The risers slip over the stock pot holders. I may post detailed instructions on making them at some point. Results from first field test melting snow: In November on Sasse Ridge, 33 degrees, raining, tent window zipped closed.† CO levels melting snow averaged 18 to 20 ppm with a maximum of 23 ppm. Without the pot lifters, very little of the heat output goes to warming the air around the stove. With the pot lifters and the stove turned up full the tent was warmed to the point where I could wear only my base layer. Normally I would have to add a couple of† layers to stay warm while melting snow at this temperature. Somewhat surprisingly, the time needed to melt my usual amount of water (~2/3 gallon) for the evening and next day was cut 20% or more. Some of the extra heat output may have been due to more complete combustion but I think the major contributor was the increase in operating temperature of the burner assembly allowing more liquid fuel per unit time to be vaporized and burned. In the shop, I measured temperatures at several points on the vaporizer from priming to smooth operation using an infrared thermometer.† Roughly,† the minimum amount of primer needed to start the stove would heat the vaporizer to about 210 deg. F, however moderate sputtering resulted.† Enough prime to get the temperature to ~240 degrees allowed the stove to start up smoothy. Without a pot on the stove and the control spindle full open and 3 pumps with the mini-pump, the stable operating temperature was ~265 deg. F.† With the pot on the stove as in the shots below, the stable operating temperature was roughly 100 degrees higher, in the range of 350 to 360† deg F.† When the pot is removed, the operating temperature gradually falls. |



|
Exposures at home: For the sake of comparison, here are a few maximum CO levels from around the house. * ∑††††† Above the chimney of a kerosene lamp with a Duplex burner: 55 ppm * ∑††††† At nose level sitting in a chair, lamp at the center of a table: 0 ppm * ∑††††† Above a pot of cold water on a DCS commercial style natural gas home range, burner on high: 38 ppm * ∑††††† At the hot air outlet of the DCS oven while heating: 67 ppm * ∑††††† At nose level standing in front of the stove: 0 ppm The kerosene table lamp:†††††† †††††††††††† †††††††††††† †††††††††††† †††††††††††† †††††††††††† †The stove and pot of water: |


|
Data from the Optimus 99 in the tent: Here is a sample of the data collected. Note the interesting finding that opening the window did not affect the maximum CO levels. Perhaps this is because a lot of the CO is escaping through the uncoated ripstop directly above the stove. |
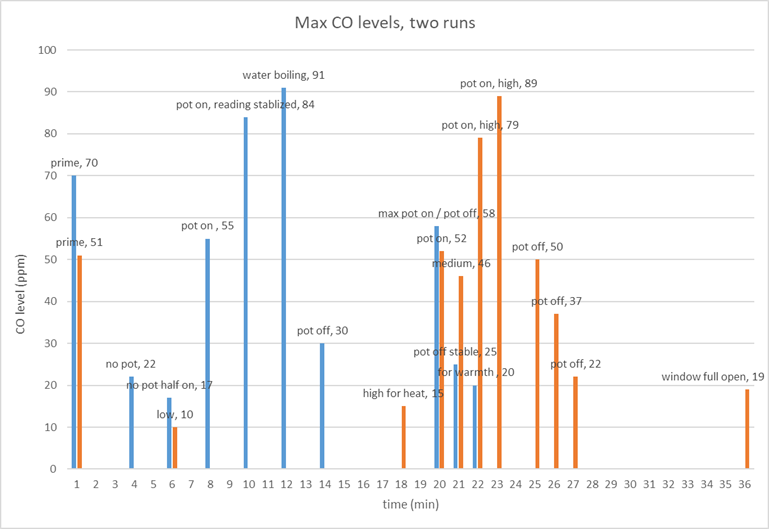
|
About me: As an emergency doc in Seattle I saw a modest number of patients with the presenting complaint of Ďpossible carbon monoxide poisoningí.† But I took that hat off long ago and here I am trying to present my recent experiments and any rough conclusions I have reached for myself for what they are worth.† Whether this applies to anyone else, I donít know.
Larry Robinson October 2021 |